The choice of a thermodynamic formulation dramatically affects modelled chemical zoning in minerals
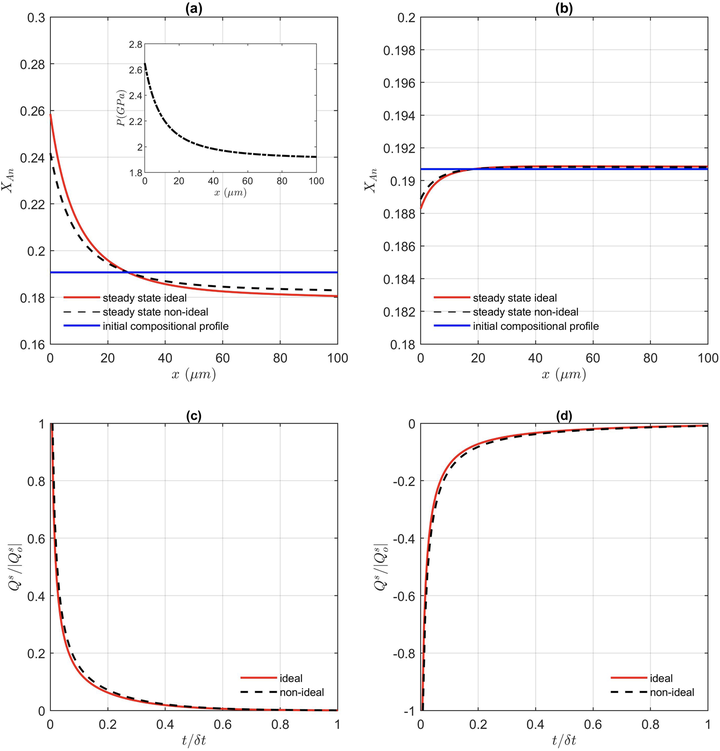 Resulting compositional profile (X Anorthite = Ca/(Ca + Na)) as a function of distance for (a) mass and (b) molar solution. The pressure profile assumed (2.7 to 1.9 GPa) is shown as inset in (a). Evolution of entropy production with time (both normalized) for the same pressure variation using the diffusion flux in (c) Mass (Eq. 2) and (d) Moles (Eq. 4). Both, ideal and non-ideal plagioclase solutions are compared.
Resulting compositional profile (X Anorthite = Ca/(Ca + Na)) as a function of distance for (a) mass and (b) molar solution. The pressure profile assumed (2.7 to 1.9 GPa) is shown as inset in (a). Evolution of entropy production with time (both normalized) for the same pressure variation using the diffusion flux in (c) Mass (Eq. 2) and (d) Moles (Eq. 4). Both, ideal and non-ideal plagioclase solutions are compared.
Abstract
Quantifying natural processes that shape our planet is a key to understanding the geological observations. Many phenomena in the Earth are not in thermodynamic equilibrium. Cooling of the Earth, mantle convection, mountain building are examples of dynamic processes that evolve in time and space and are driven by gradients. During those irreversible processes, entropy is produced. In petrology, several thermodynamic approaches have been suggested to quantify systems under chemical and mechanical gradients. Yet, their thermodynamic admissibility has not been investigated in detail. Here, we focus on a fundamental, though not yet unequivocally answered, question: which thermodynamic formulation for petrological systems under gradients is appropriate—mass or molar? We provide a comparison of both thermodynamic formulations for chemical diffusion flux, applying the positive entropy production principle as a necessary admissibility condition. Furthermore, we show that the inappropriate solution has dramatic consequences for understanding the key processes in petrology, such as chemical diffusion in the presence of pressure gradients.