Kinetics of Fe–Ti Oxide Re-equilibration in Magmatic Systems: Implications for Thermo-oxybarometry
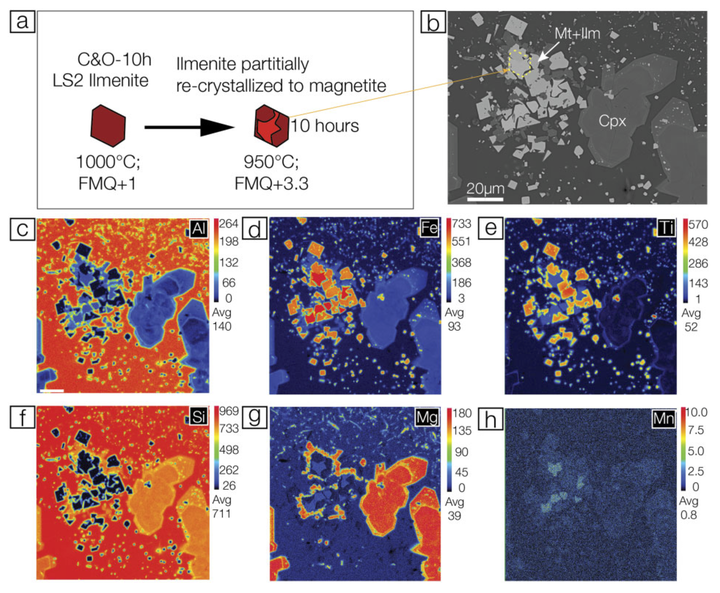 Elemental mapping of a magnetite–ilmenite cluster in the C&O-10h experiment. Panel (a) shows a schematic illustration of the ilmenite-to-magnetite recrystallization, i.e. ilmenite recrystallization into magnetite at the rim, panel (b) is the BSE image of the sample, whereas panels (c) to (h) show concentrations of Al, Fe, Ti, Si, Mg and Mn after 10 hours cooling and oxidation.
Elemental mapping of a magnetite–ilmenite cluster in the C&O-10h experiment. Panel (a) shows a schematic illustration of the ilmenite-to-magnetite recrystallization, i.e. ilmenite recrystallization into magnetite at the rim, panel (b) is the BSE image of the sample, whereas panels (c) to (h) show concentrations of Al, Fe, Ti, Si, Mg and Mn after 10 hours cooling and oxidation.
Abstract
The combined Fe–Ti oxide geothermometry and oxygen barometry is an important tool in pet- rology and volcanology. However, its appropriate application to natural magmatic systems is still challenged by poorly constrained kinetics of the re-equilibration processes between magnetite, il- menite, melt and other magmatic phases. In this study, we experimentally investigated how fast Fe–Ti oxides can re-equilibrate and how fast their compositions can adapt to changing temperature and/or redox conditions. A series of equilibrium crystallization experiments were conducted in in- ternally heated gas pressure vessels using an evolved hydrous basaltic composition. These start- ing reference experiments were conducted at 200MPa, at 900 and 1000C and at two redox condi- tions, i.e. FMQþ1 and FMQþ33. The products of the starting experiments, all containing magnetite and/or ilmenite, were then placed at a different temperature (T) and/or oxygen fugacity (f O2) for time dependent experimental series (1, 10 and 100 hours) in an attempt to check for the time needed for re-equilibration of the oxide composition. The experimental results demonstrate that both magnetite and ilmenite start to respond chemically to the changing conditions quite rap- idly in less than 1 h. The largest compositional deviations from the equilibrium compositions were observed in the runs with 1 and 10h duration. After 100h, the Fe–Ti oxide compositions were approaching the expected equilibrium values in almost all kinetic series, but still with significant deviation. Moreover, our results clearly show that the Mg/Mn ratio in magnetite and ilmenite can follow the nominal ‘equilibrium’ trend, although the Fe–Ti distribution between oxides may not have reached equilibrium. This observation implies that the use of the Mg/Mn criterion to check for the equilibrium distribution of Fe–Ti between magnetite and ilmenite should be reconsidered or at least applied with caution. The quick, within-100-hours re-equilibration of the Fe–Ti oxides at mag- matic conditions imposes limitations on the reliable application of oxide thermobarometry to nat- ural systems. We suggest that in basaltic to andesitic volcanic rocks ascending and cooling relatively slowly (more than 5 days), the compositional T–f O2 record in oxides is representative of a late evolution stage rather than the pre-eruptive conditions in a magmatic reservoir at depth. This ‘frozen’ late-stage T–fO2 record is controlled by changing element diffusion rates with cooling and degassing. Only magmatic/volcanic systems, which underwent very rapid cooling, resulting from magma ascent within minutes or few hours (e.g. Plinian eruptions), can deliver Fe–Ti oxides reflecting the pre-eruptive magmatic T–f O2 signature.